A successful regulatory professional, Robert Gadimian draws on two decades of experience working in the pharmaceutical industry. Over the years, Robert Gadimian is involved in creating drug combinations to treat diabetic foot ulcers.
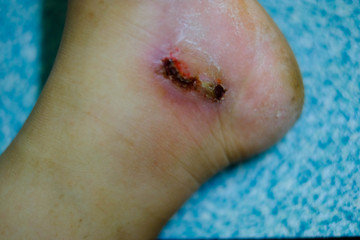
With a prevalence of 15 percent among diabetic patients, a diabetic foot ulcer is an open wound or sore that often develops around the foot’s bottom. At the initial stage, a diabetic foot ulcer can present with drainage from the foot (which makes socks wet), swelling, and sometimes redness. Diabetic foot ulcers result in infection and several other ulcer-related complications after some time, which may require hospitalization. In severe cases, patients are amputated as an intervention for complication progress. If the disease is detected early, chances of healing with treatment are possible, which will minimize the risk of infection.
A diabetic foot ulcer can occur in any diabetic patient. African Americans, Hispanics, Native Americans, and seniors, however, have higher risk factors. Likewise, diabetic patients undergoing insulin medication and patients with a diabetes-induced eye, kidney, or heart disease may develop the condition. Tobacco, alcohol, and obesity are some other risk factors. If a diabetic patient has a vascular disease, the chances of suffering complications from diabetic foot ulcers increase due to slower healing, including infection.
Generally, multiple factors can play an important role in the development of diabetic foot ulcers. Examples are foot deformities, poor circulation, irritation, duration of diabetes, and trauma. Diabetic foot ulcer patients slowly lose sensation in their foot which may cause subjecting of the foot to constant friction or pressure (2 causes of inflammation) without realizing. The inability to feel pain results from the disease’s neuropathy, which a podiatrist can assess using a painless instrument called a monofilament.
from WordPress https://ift.tt/3tOf9Y0
via IFTTT




